Download Class 12 Semiconductor Handwritten Notes PDF
Download Class 12 Semiconductor Handwritten Notes PDF
Download Class 12 Semiconductor Handwritten Notes PDF from Below⇩
It is the branch of science which deals with the electron flow through a vacuum, gas or semiconductor.
Classification of substances on the basis of conduction of electricity.
Solid
We know that, each substance is composed of atoms. Substances are mainly classified into three categories namely solids, liquids and gases.
In each solid atoms are at a definite positions and the average distance between them is constant. Depending upon the internal arrangement of atoms, solids are further divided into two groups.
1. Crystalline Solids
- The solid in which the atoms are arranged in a regular order are called the crystalline solids. In other words, we can say that in a crystalline solid.there is periodicity and regularity of its component atoms in all the directions. For example sodium chloride (common salt), diamond, Sugar, silver etc are the crystalline solids.
- Their atoms are arranged in a definite geometrical shape.
- They have a definite melting point.
- They are anisotropic, i.e., their physical properties such as thermal Conductivity refractive index etc, are different in different directions.
- They are the real solids.
2. Amorphous Solids
- The Solids in which the atoms do not have a definite arrangement are called the amorphous solids. They are also called the glassy solids. For example glass, rubber, plastic, power, etc are the amorphous solids.
- They do not have a definite arrangement of its atoms, i.e., they do not have a characteristic geometrical shape.
- They do not have a definite melting point.
- They are isotropic. i.e., their physical properties such as conductivity of heat refractive index etc, are same in all the directions.
- They are not the real solids.
- Monocrystal and Polycrystalline
- Monocrystal is a crystal in which the ordered arrangements of the atoms or molecules extends throughout the piece of solid, irrespective of its size.
- Polycrystal is a crystalline solid in which each piece of the solid has a number of monocrystals with developed faces joined together.
- The polycrystal ceramic made from PbO, ZnO and TiO are used in gas lighters and telephone receivers.
Liquid Crystals
- Some organic crystalline solid. when heated acquire fluidity but retain their anisotropic properties. They are called liquid crystals.
- Some liquid crystals like cyanobiphenyl can change the plane of polarization of light and such Liquid Crystal Displays (LCD) are used in watches and micro calculators.
Crystal Lattice
- A crystal is made up of a three- dimensional array of points such that each point is surrounded by the height bouring Points in an identical way. Such an array of points is known as bravais lattice or space lattice.
- Unit cell is the smallest unit of the crystal lattice, repetition of which in three dimensions gives rise to crystal lattice.
- The length of three sides of a unit cell are called Primitives or lattice constant represented by a, b, c. The angle between three crystallographic axis are called interfacial angles represented by α, β and γ. The primitives and interfacial angles constitute the lattice parameters of a unit cell.
- [The cubic crystal may be of the form, simple cubic (sc) lattice, the body centred cubic (bcc) lattice, the face centred Cubic (fcc) lattice.]
The coordination number is defined as the number of nearest neighbours around any lattice point (or atom) in the crystal lattice.
- (a) For sc, coordination number is 6.
- (b) For bcc, coordination number is 8.
- (c) For fcc, coordination number is 12.
- (d) For sc, atomic radius is a / 2.
- (e) For bcc, atomic is a √3 / 4.
- (f) For fcc. atomic radius is a / 2√2.
Classification of solids on the basis of conductivity
(i) Conductor:-
Conductors are those substances through which electricity can pass easily, e.g., all metals are conductors.
(ii) Insulator:-
Insulators are those substances through which electricity cannot pass, e.g., wood.
rubber, mica etc.
(iii) Semiconductor:-
Semiconductors are those substances whose conductivity lies between
conductors and insulators. e.g., germanium, silicon, carbon etc.
Energy Bands of Solids
1. Energy Band
In a crystal due to interatomic interaction valence electrons of one atom are shared by more than one atom in the crystal. Now splitting of energy levels takes place. The collection of these closely spaced energy levels is called an energy band.
2. Valence Band
This energy band contains valence electrons. This band may be PartIally or completely filled with electrons but never be empty. The electrons in this band are not capable of gaining energy from external electric field to take part in conduction of current.
3. Conduction Band
This band contains conduction electrons. This band is either empty or Partially filled with electrons.
Electrons present in this band take part in the conduction of current.
4. Forbidden Energy Gap
This band is completely empty. The minimum energy required to shift an electron from valence band to conduction band is called band gap (Eg).
Thermionic Emission
Thermionic emission occurs when a metal is heated to a high temperature, the free electrons in the metal gain kinetic energy sufficient to escape through the surface of the metal.
Thermionic Diode
The thermionic diode is a two electrode (cathode and plate) device based on thermionic
emission.
A diode allows unidirectional flow of electrons, i.e., only when the plate is positive with respect to cathode. Hence, it is also called a valve.
The triode value consists of three electrodes, e.g., cathode. plate and grid enclosed in an
evacuated glass bulb.
Grid influences the space charge and controls the flow of plate current.
[When the grid is given a negative potential with respect to cathode. It repels the electrons
escaping from the cathode and Increases the effect of space charge, at sufficientLy negative
grid potential is known as cut-off grid bias.
If the grid is given a positive potential with respect to cathode, it attracts the electrons and decreases the effect of space charge. The increasing the plate current. In this case a current flow into the circuit, thus grid modifies the function of valve.]
Grid is always kept at small negative potential with respect to cathode.
Triode can be used as an amplifier, oscillator modulator and demodulator.
An oscillator is an electronic device which generates AC voltage from DC power. It is
basically a positive feedback amplifier with infinite voltage gain.
Types of Semiconductor
(i) Intrinsic Semiconductor
A semiconductor in its pure state is called intrinsic semiconductor.
(ii) Extrinsic Semiconductor
A semiconductor doped with suitable impurity to increase its conductivity, is called extrinsic semiconductor.
On the basis of doped impurity extrinsic semiconductors are of two types
(i) n-type
An Extrinsic semiconductor doped with pentavalent impurity like As,Sb, Bi, etc in which negatively charged electrons works as charge carrier, is called n-type
semiconductor
Every pentavalent impurity atom donate one electron in the crystal, therefore it is called a doner atom
(ii) p -type
An Extrinsic semiconductor doped with trivalent impurity like Al, B, etc, in which positively charged holes works as charge carriers, is called p-type semiconductor.
Every trivalent impurity atom have a tendency to accept one electron, therefore it is called an acceptor atom.
In a doped semiconductor [ne nh = ni2]
where ne and nh are the number density of electrons and holes and ni is number density of intrinsic carriers, i.e., electrons or holes.
In n-type semiconductor, ne > > nh
In p -type semiconductor, nh > > ne
Electrical conductivity of extrinsic semiconductor is given by
σ = 1 / ρ = e (ne μe + nh μh)
where ρ is resistivity, μe and μh are mobility of electrons and holes respectively.
Note:- Energy gap for Ge is 0.72 eV and for Si it is 1.1 eV.
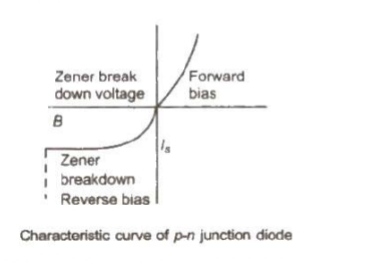
p-n Junction
An arrangement consisting a p -type semiconductor brought into a close contact with n-type semiconductor, is called a p-n junction.
The current in a p-n junction is given by
kB = Io (eeV/k BT– 1 )
where Io is reverse saturation current, V is potential difference across the diode, and kB is the Boltzmann constant.
Terms Related to p-n Junction
(i) Depletion Layer
At p-n. junction a region is created, where there is no charge carriers. This region is called depletion layer. The width of this region is of the order of 10 raise to power 6 metre.
(ii) Potential Barrier
The potential difference across the depletion layer is called potential barrier.
Barrier potential for Ge is 0.3 V and for Si is 0.7 V.
(iii) Forward Biasing
In this biasing, the p -side is connected to positive terminal and n-side to negative terminal of a battery.
In this biasing, forward current flows due to majority charge carriers. The width of depletion layer decreases.
(iv) Reverse Biasing
In this biasing, the p-side is connected to negative terminal and n-side to
positive terminal of a battery.
In this biasing, reverse current flows due to minority charge carriers.
The width of depletion layer increases.
A p-n junction diode can be utilized as a rectifier.
Zener diode, photo-diode, light-emitting diode, etc are specially designed p-n. junction diodes.
p-n Junction Diode
The current through p-n junction flow only from p toward n and not from n toward p.
The maximum voltage that a junction diode can bear without break is called zener voltage and the junction diodes possessing this voltage is known as zener diode.
Resistance of diode R = V / I
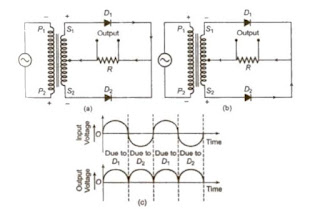
Rectifier
A device which convert alternating current or voltage into direct current or voltage is known as rectifier. The process of converting AC into DC is called rectification.
Half-Wave Rectifier
A half-wave rectifier converts the half cycle of applied AC signal into DC signal. Ordinary transformer may be used here.
Full-Wave Rectifier
A full-wave rectifier converts the whole cycle of applied AC signal into DC signal. Centre top,transformer is used here.
[Half-wave rectifier converts only one-half of AC Into DC while full wave rectifier rectifies both halves of AC input.]
Transistor
A transistor is an arrangement obtained by growing a thin layer of one type of semiconductor between two thick layers of other similar type semiconductor.
• The left side semiconductor is called emitter, the right side semiconductor is called collector and the thin middle layer is called base.
• Emitter is highly doped and base is feebly doped.
• A transistor can be utilized as an amplifier and oscillator but not a rectifier
• Maximum amplification is obtained in common-emitter configuration.
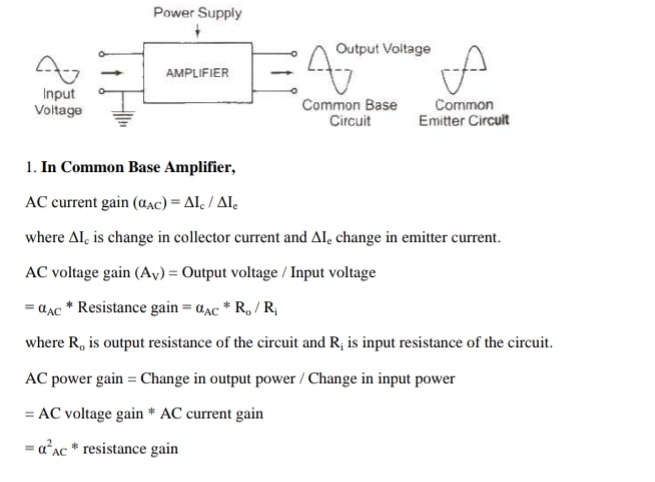
Transistor as an Amplifier
An amplifier is a device which is used for increasing the amplitude of variation of alternating voltage, current or power.
The amplifier thus produces an enlarged version of the input signal.
The general concept of amplification is represented in figure. There are two input terminals for the signal to be amplified and two output terminals for connecting the load; and’ a means of supplying power to the amplifier.
For Assignments - Semiconductor Assignment
semiconductor class 12 handwritten notes pdf, class 12 semiconductor notes pdf, semiconductor physics lecture notes pdf class 12, semiconductor notes class 12 cbse pdf, semiconductor handwritten notes class 12, class 12th semiconductor notes pdf, semiconductor class 12 notes pdf download, notes of semiconductor physics class 12 pdf, notes of semiconductor class 12 pdf, notes of semiconductor physics class 12, notes of chapter semiconductor physics class 12, notes of chapter semiconductor physics class 12










Comments
Post a Comment
Contact us if you have any queries.